Analysis: This quote does not indicate US authorities have not evaluated vaccine’s long-term effects on kids before approval
A Twitter video posted on Oct. 27, featuring a clip from a Food and Drug Administration (FDA) advisory committee meeting, claimed that a voting member said “we’re never going to learn about how safe the vaccine is until we start giving it.”
During the meeting, the members recommended the emergency use authorization of the Pfizer-BioNtech COVID-19 vaccine for children aged 5-11 in the United States.
The tweet set off a comment chain with people venting their anger towards the FDA’s decision, pointing to the quote as an indication that the FDA did not know whether the vaccine was safe for use in children.
Some went as far as to claim that this was tantamount to experimenting on kids.
At the time of writing, the initial post and the subsequent tweet together have been retweeted over 10,000 times and been viewed 1.7 million times.
Annie Lab looked into the claim and has determined that the quote is real. However, it is being used out of context.
The quote is only one part of a much larger deliberation that also included inputs from other experts about the safety of the vaccine for children. The FDA panel also weighed in on benefit-risk models with data showing the vaccine’s benefits would outweigh the risks.

Annie Lab was able to find an official video record of the meeting from the FDA’s YouTube channel. It was live-streamed on Oct. 26.
We also found the original briefing document and meeting roster from the FDA’s website.
The expert in the quoted video is Dr. Eric Rubin, an adjunct professor of immunology and infectious diseases at the Harvard T.H. Chan School of Public Health.
He is one of the 18 voting members on the Vaccine and Related Biological Products Committee (VRBPAC), an advisory committee to the FDA tasked with providing recommendations on vaccine approval.
The meeting was called to determine whether or not the FDA should approve the use of the Pfizer-BioNtech COVID-19 vaccine for use in children aged 5 to 11.
To sum up Dr. Rubin’s view, he expressed his concern over the side effects for younger groups and pointed out the benefit of inoculation might not be as good compared to protecting the elderly, while he also saw the vaccine as working and safe.
He eventually supported the approval of the motion.
The side-effect in question is myocarditis: a condition involving the inflammation of the heart muscle. It is a documented side-effect of the Pfizer-BioNtech Vaccine that particularly affects adolescent males and young adults.
The panel weighed in on a benefit-risk analysis presented by Dr. William Gruber, Pfizers’ senior vice president for vaccine clinical research and development.
Gruber presented FDA Scenario 4, which he said, “appears the better match, given the 90.7% observed clinical trial efficacy in 5 to < 12-year-olds.”
It shows that for one million fully-vaccinated kids aged 5-11, there is a risk of 22-106 cases of myocarditis occurring as a side effect as based on three datasets: one from Vaccine Safety Datalink (VSD), a longstanding initiative from the Centers for Disease Control (CDC) and Prevention which monitors and reviews the effects of vaccines, the Vaccine Adverse Effects Reporting System (VAERS) and Optum, a health care provider.
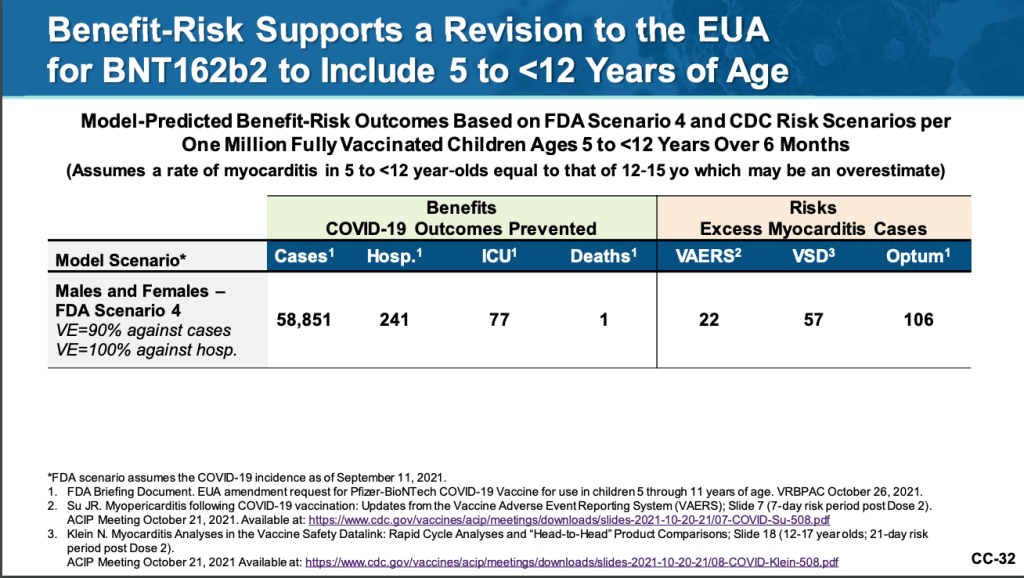
In addition, the assumed vaccine-related myocarditis incidence rate in this benefit-risk estimation for aged 5 to 11 may be conservatively overestimated, the briefing document from the FDA pointed out.
The analysis adopted the rate of myocarditis from the age group of 12-15, due to the lack of available data for the target age group. The observed systemic reaction for children at the age of 5-11 that is associated with the antigen content is lower as well.
The highest incidence of reported myocarditis in the U.S. following vaccination occurred among men aged 16-17, at a rate of 0.007%, according to a presentation by Dr. Matthew Oster, an expert from the CDC COVID-19 vaccine task force.
There have been no deaths attributed to vaccine-induced myocarditis in the U.S. as of the time of writing, and most make full recoveries after a few weeks.
Of the 18 voting members of the committee, 17 voted yes to recommend the use of the vaccine in children, including Rubin. One member abstained.
Following the voting procedure, committee members were allowed to make final remarks regarding their decision.
In his remarks, which begin here, Dr. Rubin said they would be “concerned” with the notion of a vaccine mandate for children aged 5-11.
Fellow voting member Dr. Cody Meizner shared this sentiment, with others suggesting observations should continue on potential side-effects.
Both FDA and CDC approved the emergency use authorization of the Pfizer COVID-19 vaccine on children aged 5 to 11 on Oct.29 and Nov.2, respectively, with the intended dose for children aged 5-11 being 10 µg, or one-third of the dosage given for those aged 12-15.
Vaccination for younger children in Hong Kong
Hong Kong authorities are also expected to approve the BioNTech vaccine for 5 to 11-year-olds based on the clinical trials conducted by Pfizer, according to Professor Ivan Hung who spoke on a radio program.
Hung is an expert committee member advising the government on Covid vaccines. He reportedly said only one-third of a dose will be administered and the vaccination will be crucial in case of an assumed fifth wave.
The South China Morning Post reported Sinovac has also submitted data to the HK government showing that their vaccine is safe for children as young as six months old, in a bid to seek approval for its use on younger individuals.